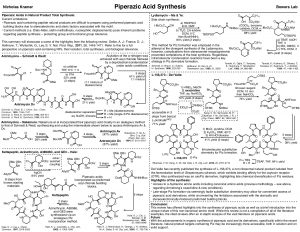
We’ve been reading the recent work of the Del Valle lab at USF and reflecting on the intriguing chemistry of what they call N-amino peptides (NAPs). The unique structural and electronic features of NAPs make them potentially useful as peptidomimetics and in SAR and drug discovery efforts. The novel N-amino group can presumably accentuate the sp2 character of the amide nitrogen while also contributing a new H-bonding prosthetic at this position; indeed, intramolecular H-bonding to the proximal amide carbonyl enhances the torsional rigidity of NAPs, stabilizing specific conformations, such as beta-sheets. Not surprisingly, nature has made extensive use of NAPs in natural products, in particular in the form of the non-proteinogenic amino acid piperazic acid (Piz), which is present in a large number of bioactive cyclic peptides.
The trick with NAPs is how to make them. Previously, the most efficient and reliable method for accessing α-hydrazino amino acid constituents of NAPs was viaa stepwise sequence from L-aliphatic amino acids, involving sequential diazotization, triflation, substitution, and saponification steps. Unfortunately, this chemistry is not compatible with side chains bearing heteroatoms or with common acid-labile protecting groups, which largely limits the scope to aliphatic amino acids. The USF group has nicely turned to the chemistry of Electrophilic Oxaziridines for N-Amination, a method pioneered by Schmitz, Collet, Vidal, and Armstrong some decades ago. With new conditions, which include aqueous NaHCO3, the group can circumvent typical by-products of this oxaziridine chemistry and broaden the scope to 19/20 canonical amino acids while boasting high conversion rates and isolated yields. Of course, the USF group has extended this chemistry to natural product total synthesis. They completed synthesis of the Piz-containing natural product, L-156,373 by means of a late stage cyclization onto NAPs prepared by their oxaziridine chemistry (Piperazic Acid Synthesis).
One lingering concern is in the coupling to the α-hydrazino amino acids. Although the late-stage chemistry used here, side-steps the steric challenge of this bond formation, there is still considerable electronic deactivation, which necessitates strong coupling agents, even in the Del Valle work. Late-stage cyclization of the Piz side chain is used in nature as well, but it is the N-N juncture that is formed via activation of an ornithine d-amino group and nucleophilic attack. One wonders if a biomimetic strategy, involving late stage N-N bond formation might leave the a-amino more accessible for coupling. Regardless, there should be exciting things to come from this family of novel amino acids. Clearly, the concise and elegant synthesis of L-156,373 demonstrates an alternate route to Piz-containing peptides, which should open the door for much extensive SAR work.
N-amino peptide conformation papers:
- Kang, C. W.; Sarnowski, M. P.; Ranatunga, S.; Wojtas, L.; Metcalf, R. S.; Guida, W. C.; Del Valle, J. R. Chem. Commun. 2015, 51, 16259-16262
- Sarnowski, M. P.; Kang, C. W.; Elbatrawi, Y. M.; Wojtas, L.; Del Valle, J. R. Angew. Chem. Int. Ed. 2017, 56, 2083-2086
- Sarnowski, M. P.; Pedretty, K. P.; Giddings, N.; Woodcock, H. L.; Del Valle, J. R. Bioorganic & Medicinal Chemistry 2017, 26, 1162-1166
Methodology:
- Kang, C. W.; Sarnowski, M. P.; Elbatrawi, Y. M.; Del Valle, J. R. J. Org. Chem., 2017, 82, 1833-1841
Total Synthesis:
- Elbatrawi, Y. M.; Kang, C. W.; Del Valle, J. R. Org. Lett. 2018, 28, 2707-2710